我们每月定期收集引用 Bioss产品发表的文献。截止目前,引用Bioss产品发表的文献共17984篇,总影响因子77329.853分,发表在Nature / Science / Cell 以及 Immunity 等顶级期刊的文献共51篇,合作单位覆盖了清华、北大、复旦、华盛顿大学、麻省理工学院、东京大学以及纽约大学等国际知名研究机构上百所。我们每月收集引用 Bioss产品发表的文献。若您在当月已发表SCI文章,但未被我公司收集,也请致电我们,我们将赠予现金鼓励,金额标准请参考“发文章 领奖金”活动页面。
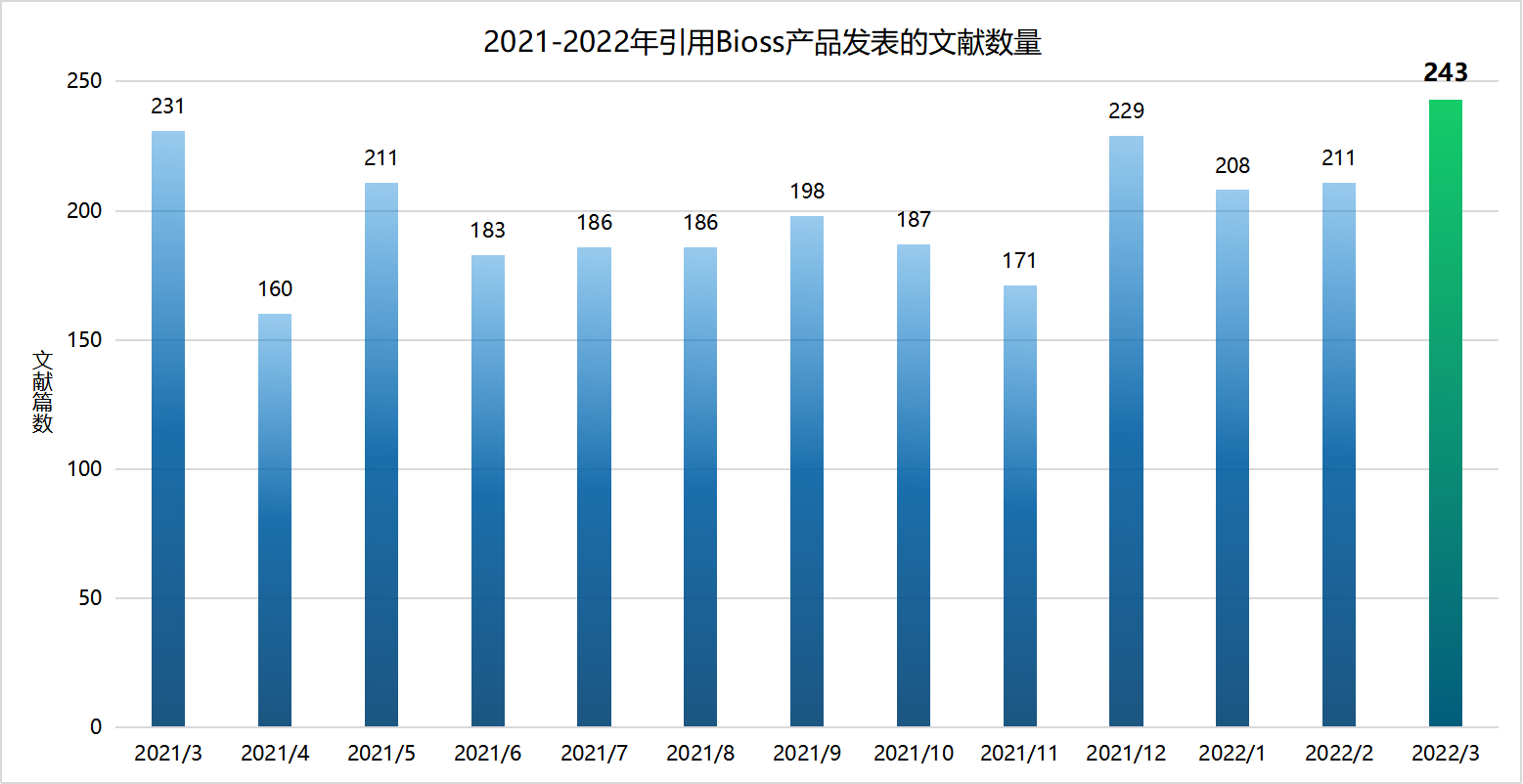
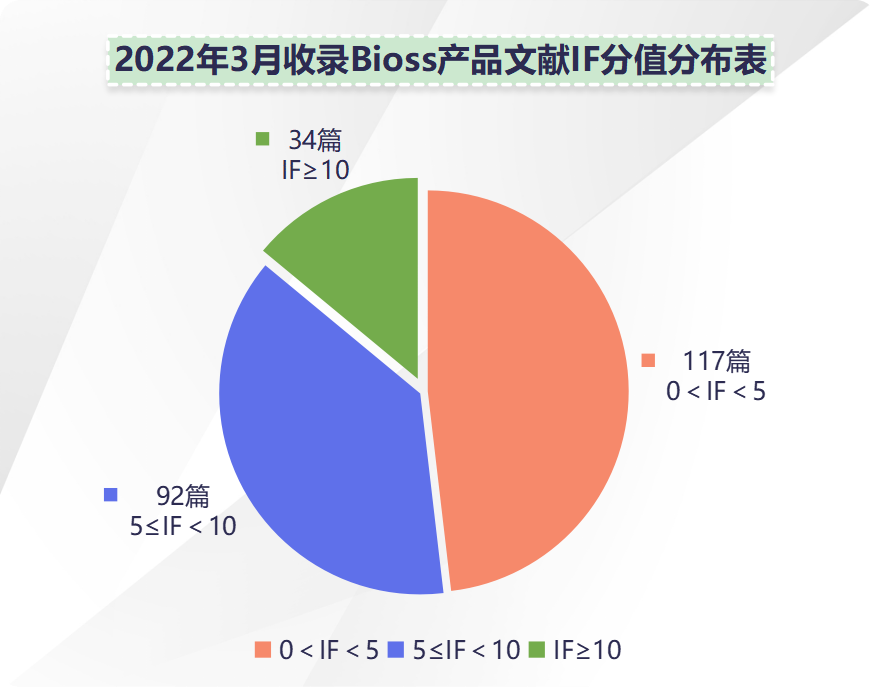
近期收录 2022年3月引用 Bioss 产品发表的文献共243篇(图一,绿色柱),文章影响因子 (IF) 总和:1587.011,30分以上文献:4篇,20分以上文献:8篇,10分以上文献:34篇(图二)。
图一
图二
本文分享来自 Nature Nanotechnology / Immunity / Cancer Cell 等期刊的8篇 IF>20的文献摘要,让我们一起欣赏这些文章吧。
文献 1
Institution : 哥伦比亚大学生物化学和分子生物物理系,莫蒂默·祖克曼心理大脑行为研究所摘要: Misfolding and aggregation of disease-specific proteins, resulting in the formation of filamentous cellular inclusions, is a hallmark of neurodegenerative disease with characteristic filament structures, or conformers, defining each proteinopathy. Here we show that a previously unsolved amyloid fibril composed of a 135 amino acid C-terminal fragment of TMEM106B is a common finding in distinct human neurodegenerative diseases, including cases characterized by abnormal aggregation of TDP-43, tau, or α-synuclein protein. A combination of cryoelectron microscopy and mass spectrometry was used to solve the structures of TMEM106B fibrils at a resolution of 2.7 Å from postmortem human brain tissue afflicted with frontotemporal lobar degeneration with TDP-43 pathology (FTLD-TDP, n = 8), progressive supranuclear palsy (PSP, n = 2), or dementia with Lewy bodies (DLB, n = 1). The commonality of abundant amyloid fibrils composed of TMEM106B, a lysosomal/endosomal protein, to a broad range of debilitating human disorders indicates a shared fibrillization pathway that may initiate or accelerate neurodegeneration.
文献 2
文献引用抗体:bs-0673R | Anti-human B7H4 pAb | OtherInstitution : 德国德累斯顿大学医学中心第一医学系摘要:Bacterial sensing by intestinal tumor cells contributes to tumor growth through cell-intrinsic activation of the calcineurin-NFAT axis, but the role of this pathway in other intestinal cells remains unclear. Here, we found that myeloid-specific deletion of calcineurin in mice activated protective CD8+T cell responses and inhibited colorectal cancer (CRC) growth. Microbial sensing by myeloid cells promoted calcineurin- and NFAT-dependent interleukin 6 (IL-6) release, expression of the co-inhibitory molecules B7H3 and B7H4 by tumor cells, and inhibition of CD8+T cell-dependent anti-tumor immunity. Accordingly, targeting members of this pathway activated protective CD8+T cell responses and inhibited primary and metastatic CRC growth. B7H3 and B7H4 were expressed by the majority of human primary CRCs and metastases, which was associated with low numbers of tumor-infiltrating CD8+T cells and poor survival. Therefore, a microbiota-, calcineurin-, and B7H3/B7H4-dependent pathway controls anti-tumor immunity, revealing additional targets for immune checkpoint inhibition in microsatellite-stable CRC.
文献 3
Institution : 中国科学院生物物理研究所感染与免疫重点实验室摘要:Tuft cells are a type of intestinal epithelial cells that exist in epithelial barriers and play a critical role in immunity against parasite infection. It remains insufficiently clear whether Tuft cells participate in bacterial eradication. Here, we identified Sh2d6 as a signature marker for CD45+ Tuft-2 cells. Depletion of Tuft-2 cells resulted in susceptibility to bacterial infection. Tuft-2 cells quickly expanded in response to bacterial infection and sensed the bacterial metabolite N-undecanoylglycine through vomeronasal receptor Vmn2r26. Mechanistically, Vmn2r26 engaged with N-undecanoylglycine activated G-protein-coupled receptor-phospholipase C gamma2 (GPCR-PLCγ2)-Ca2+ signaling axis, which initiated prostaglandin D2 (PGD2) production. PGD2 enhanced the mucus secretion of goblet cells and induced antibacterial immunity. Moreover, Vmn2r26 signaling also promoted SpiB transcription factor expression, which is responsible for Tuft-2 cell development and expansion in response to bacterial challenge. Our findings reveal an additional function of Tuft-2 cells in immunity against bacterial infection through Vmn2r26-mediated recognition of bacterial metabolites.
文献 4
[IF=30.849] Advanced Materials
Institution : 中国科学院纳米材料与纳米安全生物医学效应重点实验室
摘要:Therapeutic mRNA vaccination is an attractive approach to trigger antitumor immunity. However, the mRNA delivery technology for customized tumor vaccine is still limited. In this work, bacteria-derived outer membrane vesicles (OMVs) are employed as an mRNA delivery platform by genetically engineering with surface decoration of RNA binding protein, L7Ae, and lysosomal escape protein, listeriolysin O (OMV-LL). OMV-LL can rapidly adsorb box C/D sequence-labelled mRNA antigens through L7Ae binding (OMV-LL-mRNA) and deliver them into dendritic cells (DCs), following by the cross-presentation via listeriolysin O-mediated endosomal escape. OMV-LL-mRNA significantly inhibits melanoma progression and elicits 37.5% complete regression in a colon cancer model. OMV-LL-mRNA induces a long-term immune memory and protects the mice from tumor challenge after 60 days. In summary, this platform provides a delivery technology distinct from lipid nanoparticles (LNPs) for personalized mRNA tumor vaccination, and with a “Plug-and-Display” strategy that enables its versatile application in mRNA vaccines.
文献 5
[IF=25.841] Journal of Extracellular Vesicles
Institution : 中国科学院药物研究国家重点实验室,上海药物研究所摘要:Extracellular vesicles (EVs) have been proved a promising small interfering RNA (siRNA) delivery vehicle to mediate gene-silencing. Tumour-derived extracellular vesicles (TDEVs) as genetic exchange vectors in the tumour microenvironment, enable intercellular communication for a wide range of endogenous cargo molecules, such as RNAs and proteins. However, the oncogenic cargo of TDEVs limits their application in siRNA delivery for cancer therapy. Herein, we isolated TDEVs from hepatocellular carcinoma (HCC) cells and derived TDEV membranes by abandoning their content. Innovative TDEV membrane hybrid lipid nanovesicles (LEVs) were then fabricated by fusion of TDEV membranes and phospholipids to realize precise delivery to tumours and highly efficient transfection of siRNA. The TDEV membranes endow LEVs with ‘homing’ targeting ability, facilitating specific internalisation into parent HCC cells primarily through heparan sulfate proteoglycan-mediated pathways. Unlike conventional lipid-based nanovesicles, LEVs can bypass the endosomal degradation pathway, boost the delivery of siRNA through the Golgi and endoplasmic reticulum (ER) intracellular ‘freeway’ transportation, achieving a 1.7-fold improvement in siRNA transfection efficiency compared with liposomes. Additionally, siRNA loaded LEVs were demonstrated to enhance the antitumour efficacy in HCC bearing mice through effective gene silencing in the tumour sites. Our results highlight the potential application of the TDEV membrane-derived nanovesicles as an advanced siRNA delivery strategy for cancer therapy.
地 址: 北京市通州区马驹桥镇联东U谷西区四期67号楼
联系人: 秦
电 话: 4009019800
传 真: 010-58129612
Email:sales@bioss.com.cn